
Carbon dioxide plays an essential role in sustaining life on earth, but its excessive concentration in the atmosphere is causing climate change. CCS helps us avoid further CO2 emissions.

Carbon dioxide is made up of two oxygen atoms paired with a single carbon atom, chemically represented as CO2. Occurring naturally in its gaseous form, it is essential to life on earth because of its central role in the carbon cycle - a series of complex chemical processes that govern the metabolism of every organism on the planet. Put simply, living organisms release CO2 during respiration and terrestrial plants and marine phytoplankton use it for photosynthesis, then it returns to animals through food intake and digestion. The average concentration of CO2 in our atmosphere is 0.04%. Another of its crucial roles is to create the greenhouse effect, a natural process that traps a portion of the sun's heat on the surface of our planet, ensuring that temperatures are suitable to life, as happens in greenhouses during the winter. Without CO2, the earth's surface would be much colder and there would be no vegetation at all.
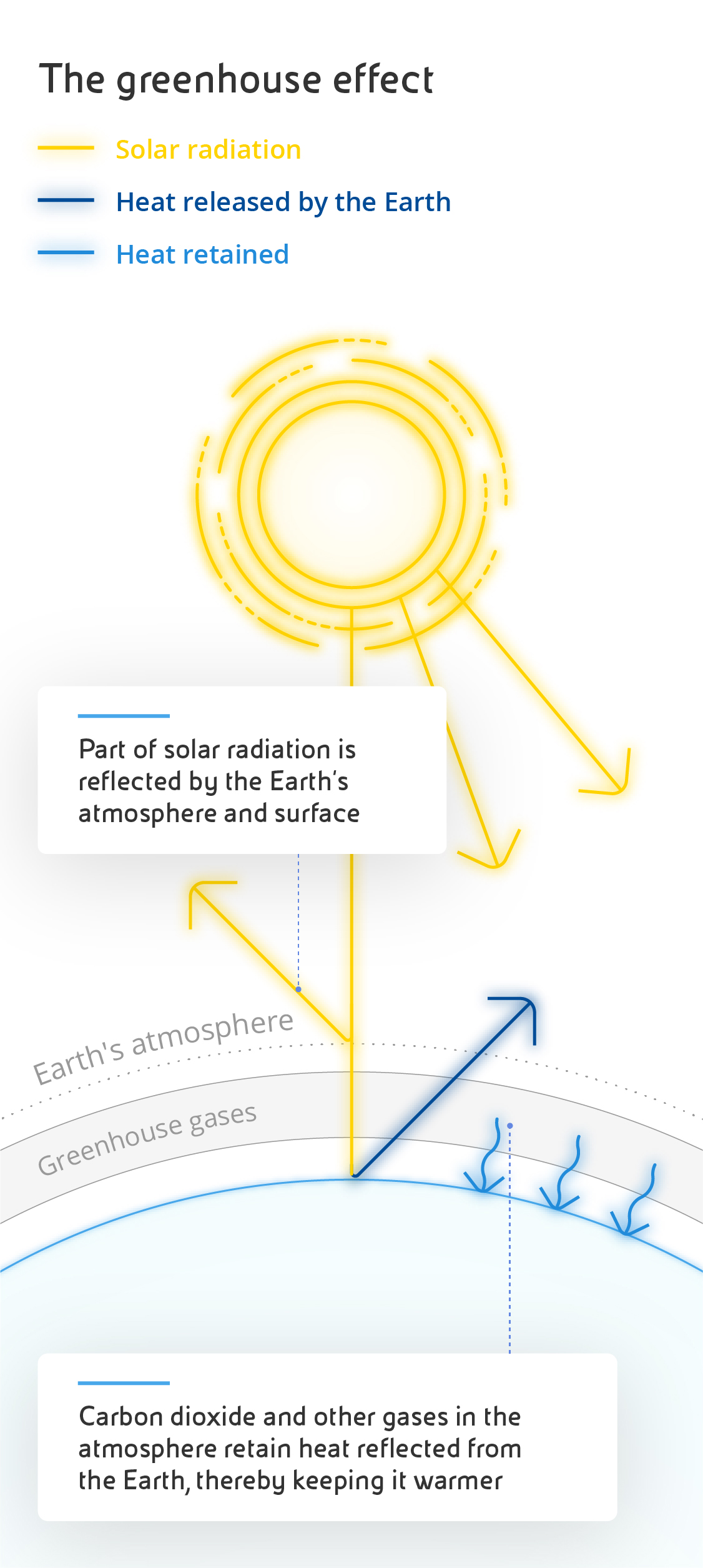
However, as CO2 levels rise, the greenhouse effect also becomes more intense, leading to an increase in global average temperatures, which in turn affects climate patterns.
Carbon dioxide plays an essential role in sustaining life on earth, but its excessive concentration in the atmosphere is causing climate change. CCS helps us avoid further CO2 emissions.
CO2 concentration in 2022 (source: NOAA - National Oceanic and Atmospheric Administration)
CO2 concentration at the end of the 18th century (source: EEA - European Environment Agency)
limit to the maximum increase in global temperatures base on the Glashow agreements
This happens because all fossil fuels contain carbon that was absorbed by living organisms millions of years ago. When these fuels are burned, carbon is released back into the atmosphere as carbon dioxide, upsetting in the space of a few decades a balance that had taken millennia to achieve. Apart from its impact on the climate, CO2 is an inert, odourless and colourless gas. It is neither flammable nor explosive, which makes it suitable for applications such as fire extinguishers. Other common uses of CO2 include the decaffeination of coffee, the production of dry ice and, of course, the preparation of carbonated drinks.
Eni and Snam are developing a carbon capture and storage (CCS) project to reduce emissions from “hard to abate” industrial facilities.
Eni and Snam are developing a carbon capture and storage (CCS) project to reduce emissions from “hard to abate” industrial facilities.